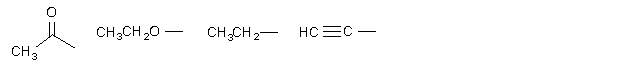
Quiz 3
Chem 226 / Dr. Rusay
Reply to the e-mail sent to you with the answers inserted into the e-mail. DO NOT alter it. DO NOT submit hard copy. E-mail submissions only will be accepted. This is an individual assignment and is to be done completely without collaboration. You may use your textbook and molecular models but no living, breathing resources.
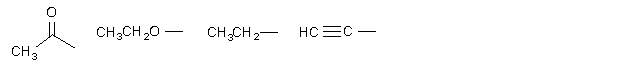
A ...................B...................... C...................... D
____________________________________________________________
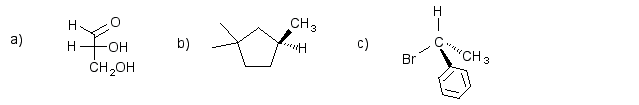
2) Give the R- or S- designation for the stereogenic center in each compound.
___________________________________________________________
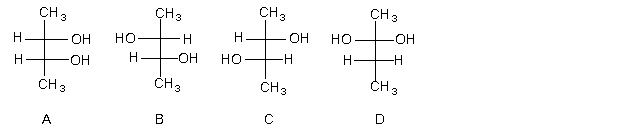
3) Consider the following compounds' respective Fischer projections.
a) Which are meso compounds? (list all)
b) Which are erythro? (list all)
c) Which are threo? (list all)
d) Which are enantiomers? (list pairs)
e) Which are diastereomers? (list pairs)
f) Which are achiral? (list all)
____________________________________________________________
4) Identify the following pairs of molecules as structural isomers, conformational isomers, enantiomers, diastereomers or identical structures.(One answer for each pair.)
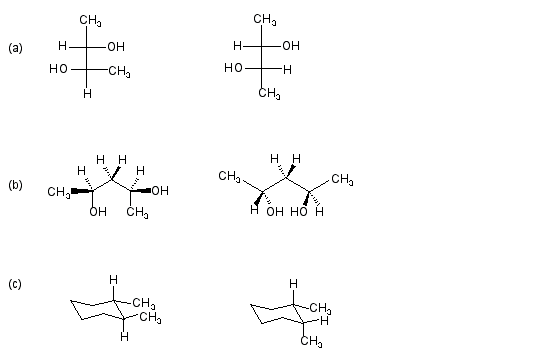
____________________________________________________________
5) Lactic acid is produced in the blood strream in large amounts when muscles are strenuously used in exercise or physical labor. There is only one stereoisomer produced, the d- isomer, which has an optical rotation of +2.6 degrees at 25o C using the sodium D line. Racemic lactic acid is also found in nature. It is produced in the fermentation of sugars and is found in tomato juice, apples, beer and wine.
A 100 mg sample of (d,l)-lactic acid was treated with an isomerase, an enzyme that stereospecifically converts the l- to the d- form. The optical rotation of the resulting product was measured to be +0.6 degrees at 25oC using a sodium source.
All of the l- form was not converted.
Consult: http://chemconnections.llnl.gov/organic/Chem226/Exams/NADH.htm